 View larger
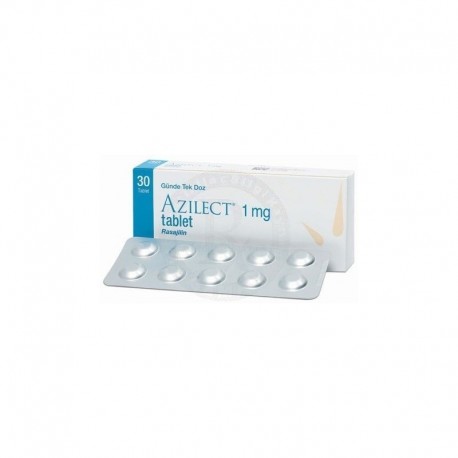
View larger Azilect (rasagiline) 1 Mg 30 Tablets
AZ4496
New product
| Quantity | Discount | You Save |
|---|---|---|
| 2 | 5% | Up to $8.40 |
| 3 | 10% | Up to $25.20 |
| 4 | 15% | Up to $50.40 |
| 5 | 20% | Up to $84.00 |
Azilect 1 Mg 30 Tablets ingredient rasagiline
Excipients
Mannitol, colloidal anhydride silica, corn starch, pregelatinized starch, stearic acid, talc.
Volume discounts
| Quantity | Discount | You Save |
|---|---|---|
| 2 | 5% | Up to $9.80 |
| 3 | 10% | Up to $29.40 |
| 4 | 15% | Up to $58.80 |
| 5 | 20% | Up to $98.00 |
More info
AZILECT 1 mg Tablet is taken orally.
Active substance
1 mg rasagiline (as mesylate).
Please read this INSTRUCTIONS carefully before using this medicine, as it contains important information for you.
Keep these instructions for use. You can need to read again.
• If you have other questions, please talk your doctor or pharmacist.
• This medicine has been prescribed for you personally, please do not give it to others.
• When using this medicine, tell your doctor that you are taking this medicine when you go to a doctor or hospital.
• Follow the instructions in this manual. Do not use high or low doses other than the recommended dose for the drug.
In these Operating Instructions:
1. What is AZILECT and what is it used for?
2. What to consider before using AZILECT
3. How to use AZILECT?
4. What are the possible side effects?
5. The storage of AZILECT is
included.
2. What is the product and what is it used for?
AZILECT 1 mg tablet contains 1 mg of rasagenin (as mesylate).
AZILECT 1 mg tablet is presented to patients in cardboard boxes in blister packages of 30 tablets.
AZILECT tablets are white-dirty white, round, flat, tapered, with yüz GIL ”on one side and yuvarlak 1 kon underneath, and the other face is flat.
AZILECT is used in the treatment of Parkinson's disease. Levodopa (another drug used in the treatment of Parkinson's disease) or with or without levodopa treatment can be used.
There is a loss of dopamine-producing cells in the brain in Parkinson's disease. Dopamine is a chemical that plays a role in the movement control of the brain. AZILECT helps increase and maintain dopamine levels in the brain.
3.How to use remover?
Instructions for appropriate use and dose / frequency of administrationas instructed by
Always use AZILECTyour doctor. If you are unsure you should check with your doctor or pharmacist.
AZILECT is taken one tablet (1 mg) once a day.
The route and method of
administration are taken orally. Can be taken with food or alone. Take tablets with sufficient amounts of liquid (eg with a glass of water).
Different age groups
Use in
children. Use of AZILECT in children and adolescents is not recommended.
Use in the
elderly No dosage adjustment is required in older patients.
Special use cases
Renal impairment Nowith
dose adjustment is required in patientsrenal impairment.
Liver impairment
should be used with caution in patients with mild hepatic impairment. In patients with mild hepatic impairment progressing to moderate hepatic impairment, its use should be discontinued. It should not be used in patients with severe hepatic impairment.
If you have an impression that the AZILECT effect is too strong or too weak, talk to your doctor or pharmacist.
If you use more AZILECT than you should use,use from
talk to a doctor or pharmacist if you have used more than you shouldAZILECT. Take the AZILECT box to show it to your doctor or pharmacist.
If you have forgotten to use AZILECT, do not
take double doses to compensate for forgotten doses.
Please take the next dose when it comes to my time.
If you end up using
AZILECT 4. What are the side effects?
As with all medicines, AZILECT may have side effects in people who are sensitive to the substances found in its contents.
The following side effects have been reported in placebo-controlled clinical trials: Side effects are defined as shown in the following categories: Very common: At least 1 of 10 patients can be seen.
Common: Less than one in 10 patients, but more than one in 100 patients.
Uncommon: Less than one in 100 patients, but more than one in 1,000 patients. Rare: Less than one in 1,000 patients.
Very rare: less than one in 10,000 patients.
Unknown (cannot be predicted from the available data)
Very common: Abnormal movements (dyskinesia)
Headache
Common: Abdominal pain
Falling
Allergic reaction
Fever
(influenza)
General feeling unwell (males)
Neck pain
Chest pain (angina pectoris)
Drowsiness and dizziness low blood pressure (orthostatic hypotension)
Appetite reduction
Constipation
Oral dryness
Nausea and vomiting
Gas
Abnormal blood test results (leukopenia)
Joint pain (arthralgia)
Musculoskeletal pain
Inflammation of the joints (arthritis)
Achieve numbness and muscle weakness (carpal tunnel) syndrome)
Weight loss
Abnormal dreams
Difficulty in muscle coordination ()
depressionDepression
Dizziness (vertigo)
Long-term muscle contraction (dystonia)
Rhinitis (colds)
Skin irritation (dermatitis)
Rash In
eyes blood (conjunctivitis)
suddenSudden urinary entrapment
Uncommon Stroke (cerebrovascular event) )
heart attack (myocardial infarction h)
Skin rash (vesiculobullous rash)
In addition, placebo-controlled in approximately 1% of skin cancer patients in clinical trials have been reported. However, scientific data suggest that Parkinson's disease is not associated with a high risk of skin cancer (not just melanoma), rather than a specific drug. If you notice any suspected skin changes, you should consult your doctor.
Parkinson's disease is associated with hallucinations and confusion symptoms. In post-marketing experience, these symptoms were also observed in Parkinson's patients treated with AZILECT.
There are cases reported in patients using one or more drugs for the treatment of Parkinson's disease, of being unable to withstand impulses, or with a severe desire to perform or do behavior that may harm themselves or others. These are called impulsive control disorders. The following adverse events were observed in patients using AZILECT and / or other drugs for the treatment of Parkinson's disease:
- Obsessive (out of the mind, out-of-mind, repetitive) thoughts or impulsive behaviors (acting without thinking about the consequences of their behavior).
- Extreme gambling passion that can have serious personal or family consequences.
- altered or increased sexual interest and behavior of yourself or others; increase in sexual desire.
- Uncontrollable and excessive shopping or spending money.
Tell your doctor if you are experiencing any of these behaviors; this will help to manage or reduce these symptoms.
2. Before using the product, do
not use
AZILECT in the following cases: Use CAREFULLY
If you have mild to moderate liver problems,
you should talk to your doctor if you notice any suspicious changes in your skin that you have not seen before.
Children:
AZILECT is not recommended for use under the age of 18 years.
Please consult your doctor if these warnings are valid for you, even at any time in the past.
Use of AZILECT with food and drink can be used withwithout
orAZILECT dishes.
Pregnancy
Consult your doctor or pharmacist before using the medicine.
Your doctor will decide on the use of medication during pregnancy, by assessing the benefits of the treatment against the possible risk to the fetus. AZILECT should not be used during pregnancy unless necessary.
If you discover that you are pregnant during your treatment, consult your doctor or pharmacist immediately.
Breastfeeding
Consult your doctor or pharmacist before using the medicine.
According to experimental data, AZILECT may adversely affect breastfeeding. Therefore, it should not be used during lactation. It is not known whether the mother's milk.
Vehicle and machine use There
is no study on the effects of vehicle and machine use. Dangerous machines, including motor vehicles, should not be used until you are certain that AZILECT is not adversely affected.
Consult your doctor before using the machine and the machine.
Important information about certain excipients in the content of AZILECT
This medicinal product contains 159.24 mg mannitol per tablet; do not require a warning due to the dose.
Use with other drugs
If you are currently using any prescription or non-prescription medicine or have recently used it, please inform your doctor or pharmacist.
Pleasebefore using with one of the following medicines with AZILECT consult your doctor.
Certain medications known as antidepressants and used in the treatment of depression (selective serotonin reuptake inhibitors (SSRIs), selective serotonin-norepinephrine reuptake inhibitors (SNRI), tricyclic or tetracyclic antidepressants)
Antibiotic ciprofloxacin used against infections
Coughing dextromethorphan
Eye droppers, to eliminate nasal congestions sympathomimetics such as nasal or oral medications (nasal and oral decongestants) and ephedrine or colds containing pseudoephedrine.
Use of AZILECT with antidepressants containing fluoxetine or fluvoxamine should be avoided.
To begin treatment with AZILECT, you should wait at least five weeks after stopping fluoxetine treatment.
To start treatment with fluoxetine or fluvoxamine, you should wait at least 14 days after discontinuation of AZILECT.
5. Storage of
AZILECT Keep AZILECT out of reach of children and in its container.
Store at room temperature below 25 ° C.
Use in accordance with expiration dates.
Do not use AZILECT after the expiration date in the packaging.